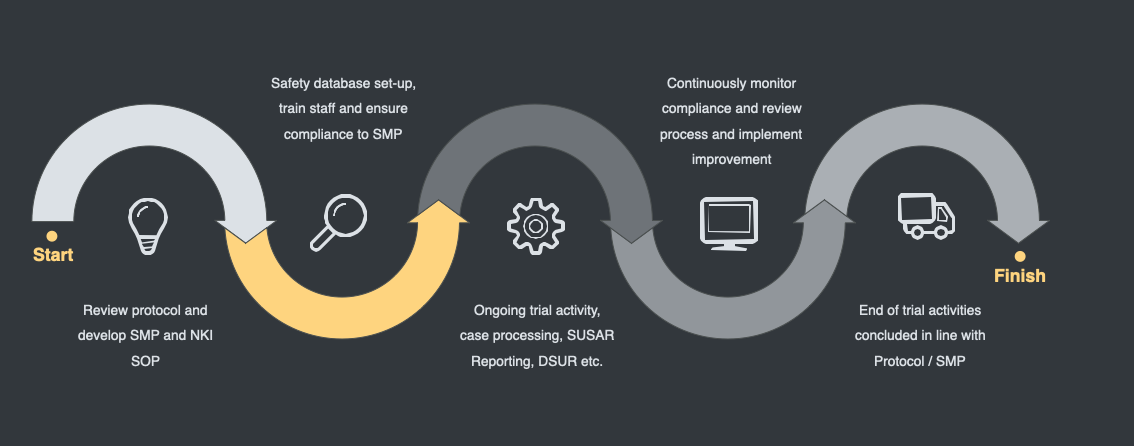
For Clinical Studies, we produce and manage Medical Monitoring and Safety Management Plans (SMP) at the request of the client and conduct the following as standard;
- Case Processing in database of sponsors choice, and support of database selection
- SUSAR reporting to authorities, ethics and investigators
- Review of IB, protocol, eCRF development, SAE forms
- Development Safety Update Reports
- Literature Review
- Development Core Safety Information
- Risk Logs
For Marketed Products, we define the process with the client and other vendors in place (e.g. Medical Information providers) to clearly outline who is responsible for each task. We support clients in a range of activities including;
- PV system set up – SOPs, databases, Safety Exchange Agreements
- Case Management – processing in database of sponsors choice
- Expedited reporting to Global Regulatory Authorities and Business Partners
- Authoring and submission of PSUR
- Signal detection and management
- Review and update of Reference Safety Information
- EU QPPV / PSMF / Eudravigilance interface
- Provision of Local Contact Person for Pharmacovigilance for Greece
- Literature Screening